MedAccred Accreditation
What is MedAccred ?
MedAccred is an industry managed supply chain oversight program that reduces risk to patient safety, assures quality products and compliance with requirements as they apply to critical processes used in the production of medical devices.
Why MedAccred?
In today’s world of global manufacturing, the supply chain is multi-tiered and geographically remote, making oversight challenging and costly. To prevent output deficiencies, critical processes and products must be validated during manufacturing to prove that they are fit for purpose, satisfy regulatory requirements and reduce overall risk
What are covered under MedAccred ?
Leveraging the industries considerable collective experience in this field, industry experts have collaborated to create a deep dive technical audit process which provides exceptional supply chain oversight in the following areas:
- Cable & Wire Harness
- Heat Treating
- Plastics
- Injection Molding
- Extrusion
- Mechanical Assembly
- Printed Boards
- Printed Circuit Board Assemblies
- Sterile Device Packaging
- Sterilization
- Welding
Processes accredited by MedAccred will continue to grow based on industry and FDA input.
Clients need to select and choose the applicable audit criteria and implement the requirements as per their plant, facility, scope, customer requirements etc
What are the Benefits ?
- Provides consistent/standardized critical process accreditation accepted by the Medical Device Industry resulting in fewer redundant onsite audits by multiple OEMs
- Conducts in-depth critical process audits that are compliant and consistent to accepted industry/technical standards and conducted by Subject Matter Experts
- Provides greater visibility of the supply chain to all levels and sub-tiers that provide critical processes, consistent with regulatory requirements (e.g. FDA, ISO 13485, MDD, etc.)
- Improves flow down of OEM requirements to sub-tier suppliers
- Medical device industry-accepted and consistent technical requirements leading to process discipline, greater operational efficiency and continuous improvement resulting in higher quality and lower overall cost.
How does the MedAccred process work?
The Performance Review Institute (PRI) administers the MedAccred program. PRI will schedule an audit and assign an approved auditor who will conduct the audit against an industry agreed standard using an industry agreed checklist. At the end of the audit, any non-conformity issues will be raised and non-conformance reports issued. PRI will administer closeout of the non-conformance reports and upon completion will present the completed audit package to a ‘special process’ task group made up of members from industry that will review it and vote on its acceptability for approval. Accreditation is granted when all nonconformances are closed.
What type of preparation is required for a MedAccred audit?
PRI/MedAccredrecommends using the applicable audit checklists to conduct a thorough self-audit. Correcting Nonconformances identified and serious preparation directly affect cycle time of processing the audit report. Staff Engineers are available at PRI to discuss any questions that suppliers may have regarding audit criteria questions.
How are the audits conducted on site?
MedAccredaudits follow general auditing protocol with an in-briefing meeting and an exit or out-briefing meeting. Auditors identify any/all nonconformances on a daily basis. The auditor will contact the supplier prior to the audit to determine the audit plan so as to disrupt the supplier operations as little as possible.
How long does the audit take?
MedAccredaudits generally take two (2) – five (5) days to conduct. The audit length is based upon the scope of the audit (number of checklist, processes, etc.).
Steps involved in MedAccred Accreditation
- Visit www.eauditnet.com, register and obtain the applicable audit check lists
- Conduct the GAP analysis and prepare an action plan for the GAP’s
- Review the existing process, documents and update inline with MedAccred Audit Criteria
- Conduct the Job Audit and Take necessary corrective action
- Get the quote for MedAccred Audit
- Schedule the MedAccred Audit
- Pay the Audit fees
- MedAccred Audit
- Closure of NC’s (In case of any NC)
- Grant of Accreditation
Pre-Requisite
Client shall have a valid Quality System like ISO 13485.
Who mandates MedAccred?
An increasing number of medical device companies are actively participating in the MedAccred program. The below are just a few of the companies actively involved
Abbott
Applied Thermal Technologies
Baxter Healthcare
Bayer
BD
Benchmark Electronics, Inc.
BMP Medical
Bodycote
Boston Scientific Corporation
DJO Global
Flex
GE Healthcare
Global Technologies
GW Plastics
Hansen Balk
Harterei Gerster
Industrial Metal Finishing
Johnson & Johnson
Kimball Electronics
Lake City Heat Treating
Mack Molding
Medtronic
Metalworx Inc
Microtech Welding
Midwest Thermal-Vac
MTD Micro Molding
Paragon Medical
Paulo Products
Philips
Plastic Plus Technology
Plexus Corp.
Quality Tech Services, LLC “QTS”
Sanmina
SINBON Electronics
Solar Atmospheres
Sterigenics
STERIS
Steri-Tek
Stryker
Techmetals
Tecomet
Vac-Met, Inc.
Zeus
Zimmer Biomet
Who are all certified for MedAccred?
From Asia
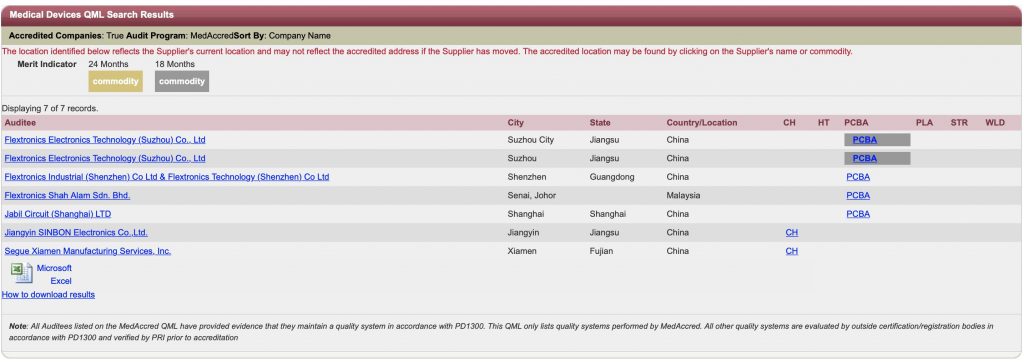
No one from India Yet… (As on August 2019)
The details are available in eauditnet.com


